The various methods of preventing electrolysis are called methods of cathodic protection.
In order to avoid sustaining damages to the installations it is imperative that we apply cathodic protection, which is achieved by placing a mass of active metal in the same environment where corrosion takes place. The primary and most cost effective active metals are zinc, aluminium and magnesium.
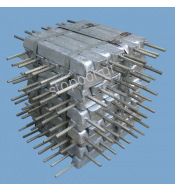
One of the most widespread methods involves the immersion in liquid or the insertion in soil of a sacrificial anode, through which certain active metals are placed in the same environment of an electrolytic cell where the phenomenon of electrolysis occurs.
These metallic items made of active metals are called anodes.
The anodes are sacrificed releasing their mass in the form of ions which are transferred towards the less active metallic mass, i.e. the various installations or constructions.
This is the reason why this particular method is called cathodic protection through sacrificial anode.
Its applications include the shipping industry and in general plumbing installations with pipelines containing water.
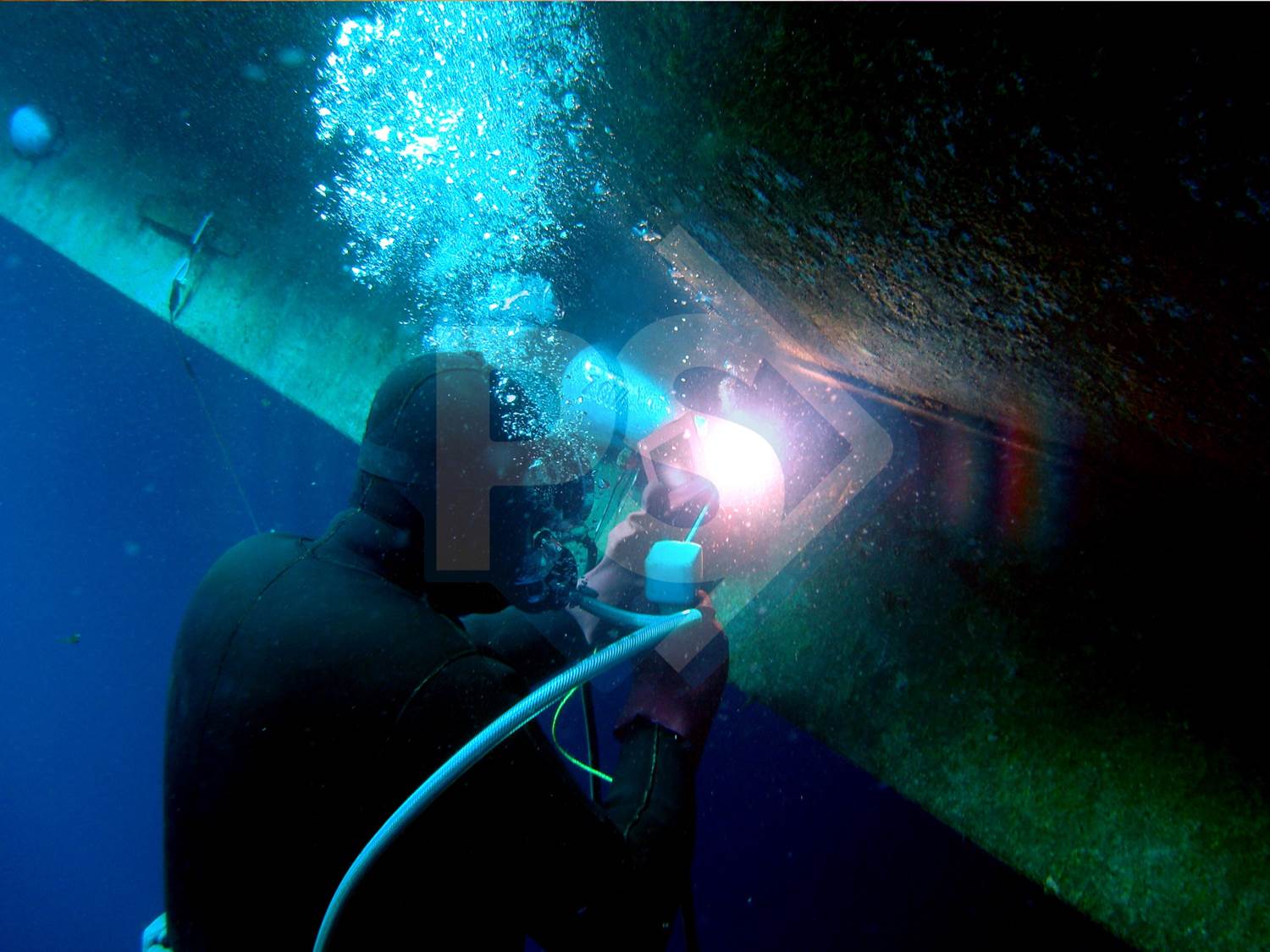
As far as the shipping industry is concerned, the most common approach is the placement of zinc or aluminium mass on the sides of the ship as well as on any surfaces which come in direct contact with water.
As regards the rest of the cases, a single mass of magnesium is inserted in the soil after being connected to the installation through a cable.
The main disadvantage of this method is the fact that it requires multiple insertion points because of the limited range of cathodic protection it can offer. Another drawback is the residue materials of the sacrificial anode, which are created and subsequently diffused into the pipelines causing damage to the fitting plumbing components. Also the intensity or efficiency of the cathodic protection offered by conventional anodes is not consistent throughout their lifespan. As a matter of fact, it is declining due to the gradual reduction in their mass. Insertion in the soil is not applicable in most installations which remain unprotected.
The other method of cathodic protection uses impress current, essentially imposing electrical current on the metals which are subjected to electrolysis, a process which requires an external power source.
This method of impress current is more efficient compared to the previous one but it requires network installation and fitting, as well as prior planning and electrochemical field study by specialized personnel. Moreover, the high cost is rather prohibitive for small or medium sized installations. Lastly, constant monitoring by a specially trained technician is required.
![Λογότυπο Eταιρίας [302xX] Λογότυπο Eταιρίας [302xX]](images/banners/1463483840_small.png)
![Πάνω Frame [668xX] Πάνω Frame [668xX]](images/banners/1450793428_logo.png)